Quality & Compliance
For Prix Pharmaceutica, quality is not just a standard — it’s a commitment woven into every stage of our operations. From selecting internationally renowned partners to delivering products across Pakistan, every step is guided by strict regulatory and safety protocols.
We adhere to the highest benchmarks of compliance, ensuring that every imported product aligns with national and global veterinary health standards.


Our Safety & Quality Protocols
DRAP-Registered and Approved: All imported products are registered with the Drug Regulatory Authority of Pakistan (DRAP), ensuring regulatory compliance and legal distribution.
Cold Chain Transport (Where Required): Temperature-sensitive products are shipped and delivered under strict cold chain management to preserve efficacy and safety.
GMP-Compliant Storage: Our warehousing systems follow Good Manufacturing Practice (GMP) standards, ensuring hygienic, organized, and secure storage conditions.
Full Batch Traceability: Each product batch is carefully logged and tracked across the supply chain, ensuring transparency and accountability from origin to end user.
Quality Assurance Certifications
ACS Registrars Pakistan
(ISO Certificate)


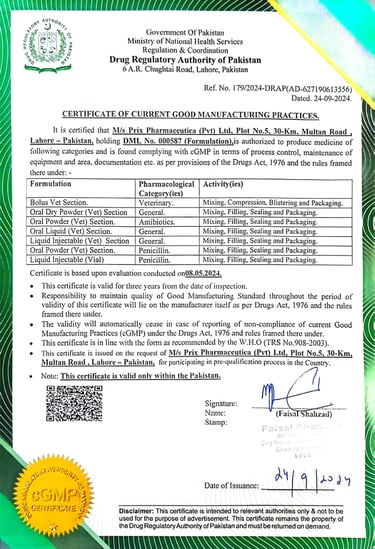
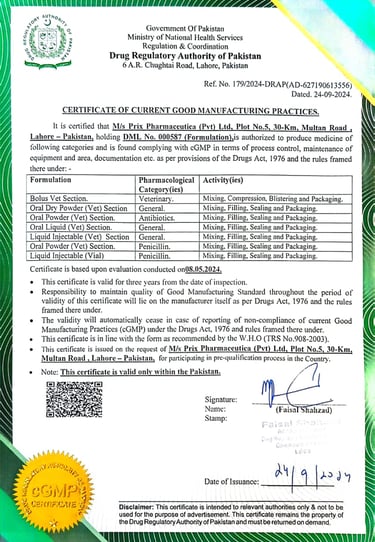


Drug Regulatory Authority
(cGMP Certificate)
Government of Pakistan
(License to Manufacture)

Contact Us
Reach out for inquiries about our veterinary pharmaceutical imports.


